Developing next generation diagnostics and immunoprofiling methods using digital pathology and AI
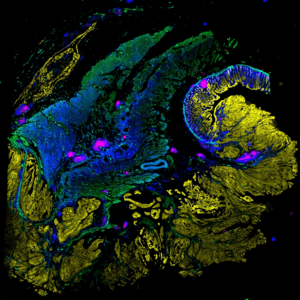
The LSP has developed new highly multiplexed imaging methods and computational tools that enable deep molecular interrogation of tumor-immune interactions and the development of next-generation diagnostics for precision cancer care.
The development of a precise, patient-centered, approach to medicine is critically dependent on being able to more precisely diagnose disease and determine which patients are most likely to benefit from a specific therapy. In oncology, disease diagnosis and management are dominated by information acquired by pathologists using histology, the examination of tissue and tumor sections in the light microscope. Despite a growing body of knowledge and the increasing impact of genetic information, the practice of pathology remains largely pre-digital. The LSP is at the forefront of developing a new generation of highly-multiplexed tissue imaging methods that can acquire precise single cell molecular information in histological specimens and fuse this with other types of genetic and single-cell data. This approach is fundamentally changing our understanding of tumor immune-interaction and the ways in which therapeutic interventions can be tailored to specific tumor types. The development of AI algorithms and large public databases (as part of the NCI Cancer Moonshot Program) is a key part of this program and we are optimistic that we will have novel methods in clinical use within two to three years. The LSP created the Harvard Tissue Atlas Project in this area as well as the Gray BRCA Atlas and the Ludwig Tumor Atlas. Our tissue imaging projects are actively recruiting staff, fellows, and students interested in cancer biology, immunotherapy, microscopy, optical physics, machine learning/AI, and cloud computing.
Associated with:
The HMS PCA Center collects and analyzes highly multiplexed single cell data on early melanomas to better understand factors that promote their progression. Part of the NCI Human Tumor Atlas Network (HTAN).
The Harvard Tissue Atlas is developing methods to precisely profile the microenvironments of diverse human tumors. Supported by the NIH, Ludwig Cancer Research Foundation, the Bill and Melinda Gates Foundation, the Gray Foundation, and the Rossy Foundation.
The Center for Cancer Systems Pharmacology (CCSP) studies the responsiveness and resistance of human tumors to anticancer drugs as well as the adverse effects that they cause. Part of the National Cancer Institute Center for Cancer Systems Biology Consortium.
Selected Publications:
Liu D, Lin J-R, Robitschek EJ, Kasumova GG, Heyde A, Shi A, Kraya A, Zhang G, Moll T, Frederick DT, Chen Y-A, Wang S, Schapiro D, Ho L-L, Bi K, Sahu A, Mei S, Miao B, Sharova T, Alvarez-Breckenridge C, Stocking JH, Kim T, Fadden R, Lawrence D, Hoang MP, Cahill DP, Malehmir M, Nowak MA, Brastianos PK, Lian CG, Ruppin E, Izar B, Herlyn M, Van Allen EM, Nathanson K, Flaherty KT, Sullivan RJ, Kellis M, Sorger PK, Boland GM. Evolution of delayed resistance to immunotherapy in a melanoma responder. Nat Med. 2021 Jun;27(6):985–992. PMCID: PMC8474080.
Nirmal AJ, Maliga Z, Vallius T, Quattrochi B, Chen AA, Jacobson CA, Pelletier RJ, Yapp C, Arias-Camison R, Chen YA, Lian CG, Murphy GF, Santagata S, Sorger PK. The Spatial Landscape of Progression and Immunoediting in Primary Melanoma at Single-Cell Resolution. Cancer Discov. 2022 Jun 2;12(6):1518–1541. PMCID: PMC9167783.
Schapiro D, Sokolov A, Yapp C, Chen YA, Muhlich JL, Hess J, Creason AL, Nirmal AJ, Baker GJ, Nariya MK, Lin JR, Maliga Z, Jacobson CA, Hodgman MW, Ruokonen J, Farhi SL, Abbondanza D, McKinley ET, Persson D, Betts C, Sivagnanam S, Regev A, Goecks J, Coffey RJ, Coussens LM, Santagata S, Sorger PK. MCMICRO: a scalable, modular image-processing pipeline for multiplexed tissue imaging. Nat Methods. 2022 Mar;19(3):311–315. PMCID: PMC8916956.
Lin JR, Wang S, Coy S, Chen YA, Yapp C, Tyler M, Nariya MK, Heiser CN, Lau KS, Santagata S, Sorger PK. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell. 2023 Jan 19;186(2):363-381.e19. PMID: 36669472
Lin JR, Chen YA, Campton D, Cooper J, Coy S, Yapp C, Tefft JB, McCarty E, Ligon KL, Rodig SJ, Reese S, George T, Santagata S, Sorger PK. High-plex immunofluorescence imaging and traditional histology of the same tissue section for discovering image-based biomarkers. Nat Cancer. 2023 Jul;4(7):1036–1052. PMCID: PMC10368530