Clinical trials

LSP investigators are improving the success of cancer clinical trials by integrating precision medicine techniques and studying effective preclinical and clinical trial design.
Clinical trials are core to all medical advances, yet over 90% fail. LSP researchers seek to understand why trials fail to improve how future trials are designed and interpreted.
A major goal of this work is to understand why patients with similar disease respond differently to the same drug. In one study, we found that when patients are treated with two therapies simultaneously, each patient benefits from the drug with the strongest effect for them, not from a synergistic interaction of both drugs. We are using this insight to design preclinical trials that better capture this patient heterogeneity. We have also curated patient-level data from clinical trials (clinicaltrials.io) so other researchers can access existing trial data and test new hypotheses.
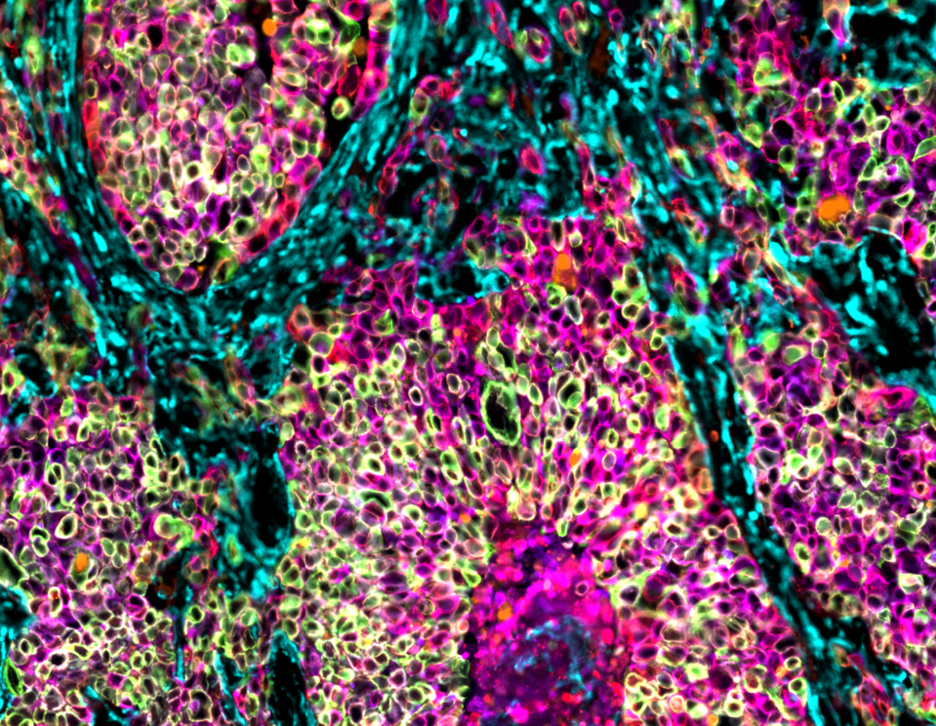
LSP researchers work closely with physicians to bring the latest tissue profiling methods into clinical trials. We recently incorporated spatial profiling into two trials, providing insight into how the immune system responds to treatment. We are also identifying new potential biomarkers and advancing computational pathology methods, which can be used to stratify patient populations based on specific tumor features and ensure that each patient gets the best treatment for their disease. Together, these efforts aim to increase the success of preclinical compounds in clinical trials and improve our ability to match patients to the ideal treatment.